新闻
-
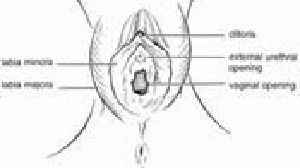
一名实施女性生殖器割礼(female genital mutilation,FGM)的伦敦产科医师被无罪开释
在一项存在争议的审判中,一名在伦敦工作的产科实习医师Dharmasena被无罪开释,他被控告对一名已进行过女阴环切术的妇女进行了FGM。当时他在为该妇女进行分娩后的阴道修补,据说在此过程中对该妇女进行了再次阴部缝合。此案引发多方批评,皇家妇产科学院校长亦给出了自己的意见,他认为这一无罪开释判决是合理的:“这是在错误的时间对错误的人所作出的错误起诉。该案在诉讼期间引起了极大的愤怒,牵涉到对患者的同情和对该实习医师的关注。难以理解一件光提起诉讼就花费了一年时间的案子是如此的站不住脚,以至于陪审团在30 分钟内就将其驳回。”
-

婴儿出生时被意外调换的两个法国家庭得到赔偿
法国南部城市格拉斯的一家法院已要求戛纳一家产科诊所向两个家庭支付近200万欧元的赔偿金,其原因是该诊所在20年前误将这两个家庭刚出生的婴儿进行了调换。这两名女婴当时在同一保育箱中接受黄疸治疗。她们的母亲在当时曾怀疑自己的女儿被调换,但诊所坚称没有问题。在10年之后,其中一个家庭因始终有所怀疑而通过DNA检验证实了所抚养的并非亲生女儿。两个家庭决定不再将女儿重新调换回来,但向法院起诉该诊所要求予以赔偿。
-
WHO calls for all trials to be registered.
On 14 April 2015 the World Health Organisation issuedits firmest statement yet on the need for clinical trials registration. “Beforeany clinical trial is initiated (at any Phase) its details are to be registeredin a publicly available, free to access, searchable clinical trial registrycomplying with WHO’s international agreed standards. The clinical trialregistry entry should be made before the first subject receives the firstmedical intervention in the trial.” The statement says that all completed orsuspended trials must be reported both in the peer reviewed literature and onthe trials registry site. There are no exceptions. The statement has beenwidely welcomed by researchers.
-
EU approves 9 valent HPV vaccine
The European Medicines Agency has recommended approval for the new Gardasil9 vaccine, active against nine types of human papillomavirus (HPV). The vaccine isalready approved in the US. It adds five more HPV types (31, 33, 45, 52, and58) to the four (6, 11, 16, and 18) currently covered by existing vaccines.
About 90% of cervicalcancers are believed to be caused by the nine HPV types. The vaccine isindicated for girls and young women aged 9 through 26 years and for boys aged 9through 15 years
-

向MDG 5前进
孟加拉国是一个发展相对落后的国家,正努力达成千年发展目标(MDG)5,即自1990年起将产妇死亡率降低75%。按计划该目标应于明年达成,孟加拉国不仅有可靠的数据,而且看起来正在达成目标的道路上前进。
EJOG原站链接:http://obstetricsgynecology.eu/news/progress-towards-mdg-5
-
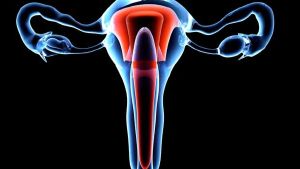
世界首例子宫移植后活产


